EXTRACTING
IMMENSE POWER
FROM THE
TINIEST SOURCE
The most abundant and lightest chemical element in
the universe has the capacity to revolutionize power.

Hydrogen – the simplest and lightest chemical element in the universe – has re-emerged as a potential powerful energy vector. It has long been shelved as a viable means to store energy due to the enormous amount of energy required to produce it, but rapidly evolving technology is making hydrogen production much more efficient.
When hydrogen gas (H2) reacts with oxygen to form water, the energy released is significant; this released energy forms the basis of hydrogen fuel technology.
A significant benefit of hydrogen power is that the only waste product is water. Unlike hydrocarbon combustion, there are no polluting emissions from hydrogen power generation. A primary drawback, however, is that hydrogen is rarely found naturally in its pure form and therefore must be produced or extracted from other molecules. To produce hydrogen in this way requires vast amounts of energy.
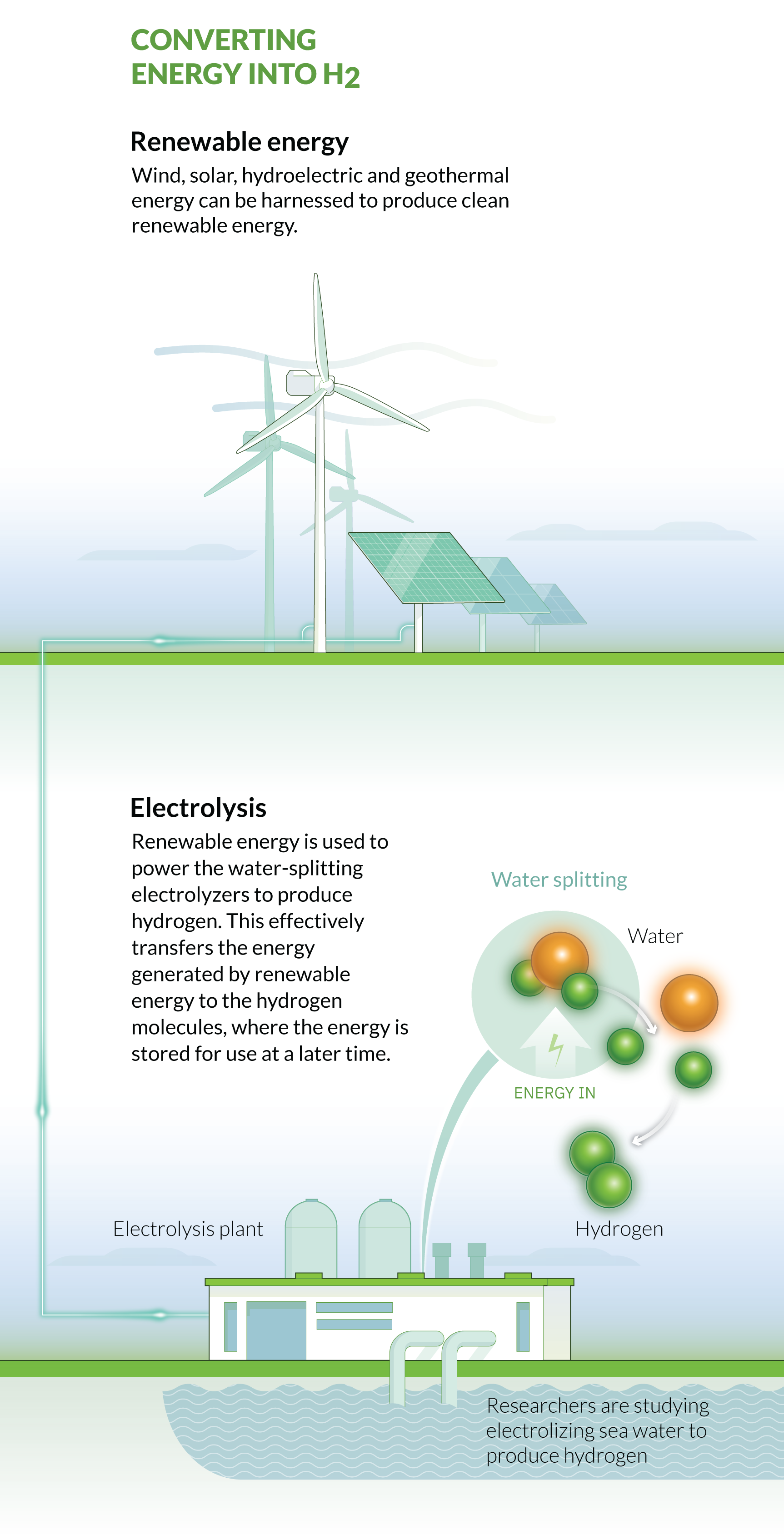
In recent years, as the cost of renewable energy has decreased, hydrogen can now be produced from renewable energy sources in an almost carbon-neutral and economically viable way. Hydrogen produced in this way is known as “green hydrogen,” and it offers a means to “store” the power produced from renewable sources, providing a solution to renewable energy’s primary disadvantage — how to fill the energy gap when renewable energy sources are not available or able to produce energy, for example at night or when there is no wind. KAUST scientists are researching ways to improve the different facets in the life cycle of hydrogen fuel and define how this fuel can address a great challenge of our time: how to power the future.
PRODUCTION
Hydrogen can be generated in several ways, but not all are carbon neutral. For instance, gray hydrogen is produced using either natural gas or coal, which produces significant carbon emissions.
Green hydrogen is almost entirely carbon neutral, although it does have some minor associated carbon emissions, such as during solar panel production. Green hydrogen is produced from water splitting — the process of splitting water molecules (H2O) into hydrogen and oxygen. Typically, this is through a process called electrolysis, which uses electricity produced exclusively from renewable sources to split the water molecule.
Until recently, the amount of electricity required to split water via electrolysis was enormous, with a low efficiency, but technological advances in electrolysis and the decreasing costs of renewables in recent years have made the process more efficient and cost effective. As the cost of hydrogen production continues to reduce, green hydrogen is becoming more cost-competitive than traditional carbon-based fuels. For instance, the production costs of green hydrogen are expected to match the costs of gray hydrogen as soon as 2030.
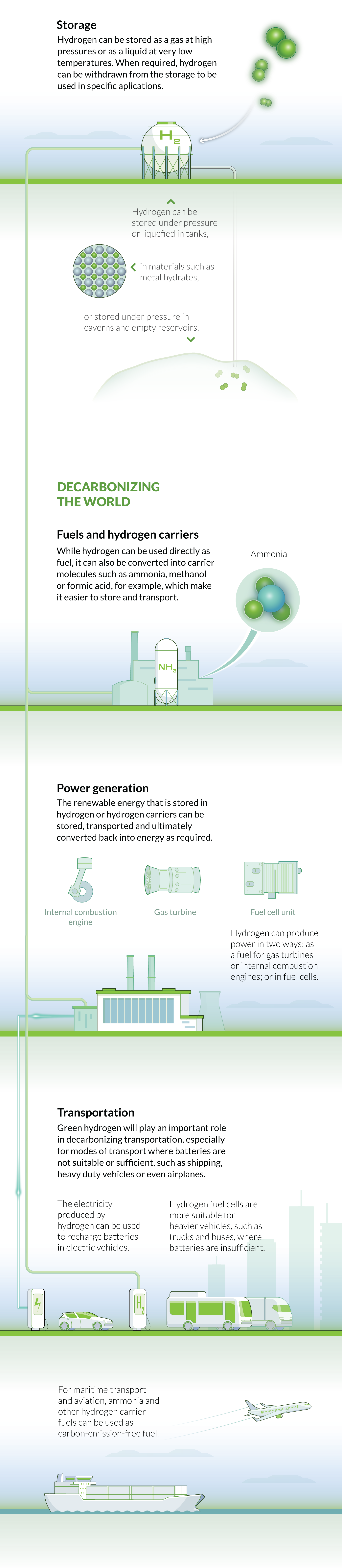
STORAGE
One of the major limiting factors of renewable energy is how to store the generated energy for later use. Battery storage – currently the most common method of energy storage - is limited to certain applications, such as powering electric vehicles, because battery technology is not yet capable of storing enough power to meet all our energy needs. Like batteries, hydrogen can also be used as a way to store energy for later use.
However, the physical storage of hydrogen molecules is difficult because it is the lightest and simplest of all elements, and this presents one of the key obstacles prohibiting its widespread adoption. Hydrogen can be stored either as a liquid or a gas, but both have inherent difficulties. For instance, hydrogen gas must be stored in high-pressure tanks, whereas the liquid form requires cryogenic temperatures (down to -252.8oC).
One promising method is through chemical or materials-based storage. Hydrogen molecules can be bound to other chemical compounds or metals at relatively low temperatures, and this can be reversed at high temperatures to release the hydrogen when it is needed. Materials-based storage involves adsorbing hydrogen to solids, such as metal hydrides, carbon-based materials and high-surface-area sorbents. Researchers are also investigating the potential for storing hydrogen gas in large underground caverns.
DISTRIBUTION
Some countries will be better equipped to produce hydrogen fuel, primarily depending on their capacity to generate renewable energy.
Saudi Arabia has potential to be a world leader in solar power generation due to its high solar exposure in addition to abundant available land that could be used for large-scale solar power and electrolysis plants. Just like the plentiful oil reserves in the Kingdom, hydrogen fuel produced here will need to be transported across the globe to nations that lack ample renewable energy resources to produce their own hydrogen.
Distribution of hydrogen will require specialized infrastructure capable of transporting the hydrogen fuel, such as pipeline networks or shipping. Such large-scale infrastructure will require substantial global and cross-border investment due to the high initial capital costs. Moreover, producing large amounts of hydrogen at a single electrolysis plant, from which the hydrogen fuel can be distributed, is considered more economically viable than several small-scale production facilities.
USE
Hydrogen can provide power in two ways: by direct reaction with oxygen in a fuel cell to produce electrical power, or by burning hydrogen in an internal combustion engine or a gas turbine (just like a conventional fossil fuel).
The choice of energy conversion is related to how the energy is to be used. Fuel cells, which convert the stored chemical energy in hydrogen into electrical energy, have the greatest potential for smaller-scale purposes, such as providing power to homes, businesses and hospitals. Unlike batteries, hydrogen fuel cells do not need to be periodically “recharged”; they will continue to operate normally as long as hydrogen fuel is supplied.
Using hydrogen in gas turbines to generate power is better suited to large-scale power generation because they are currently capable of producing 10-100 MWs of power. With the required investment in infrastructure, current conventional gas turbines could be converted into producing hydrogen gas with some modifications to the combustor design. Fully hydrogen-powered turbines are currently being developed by companies such as Mitsubishi, GE and Siemens.
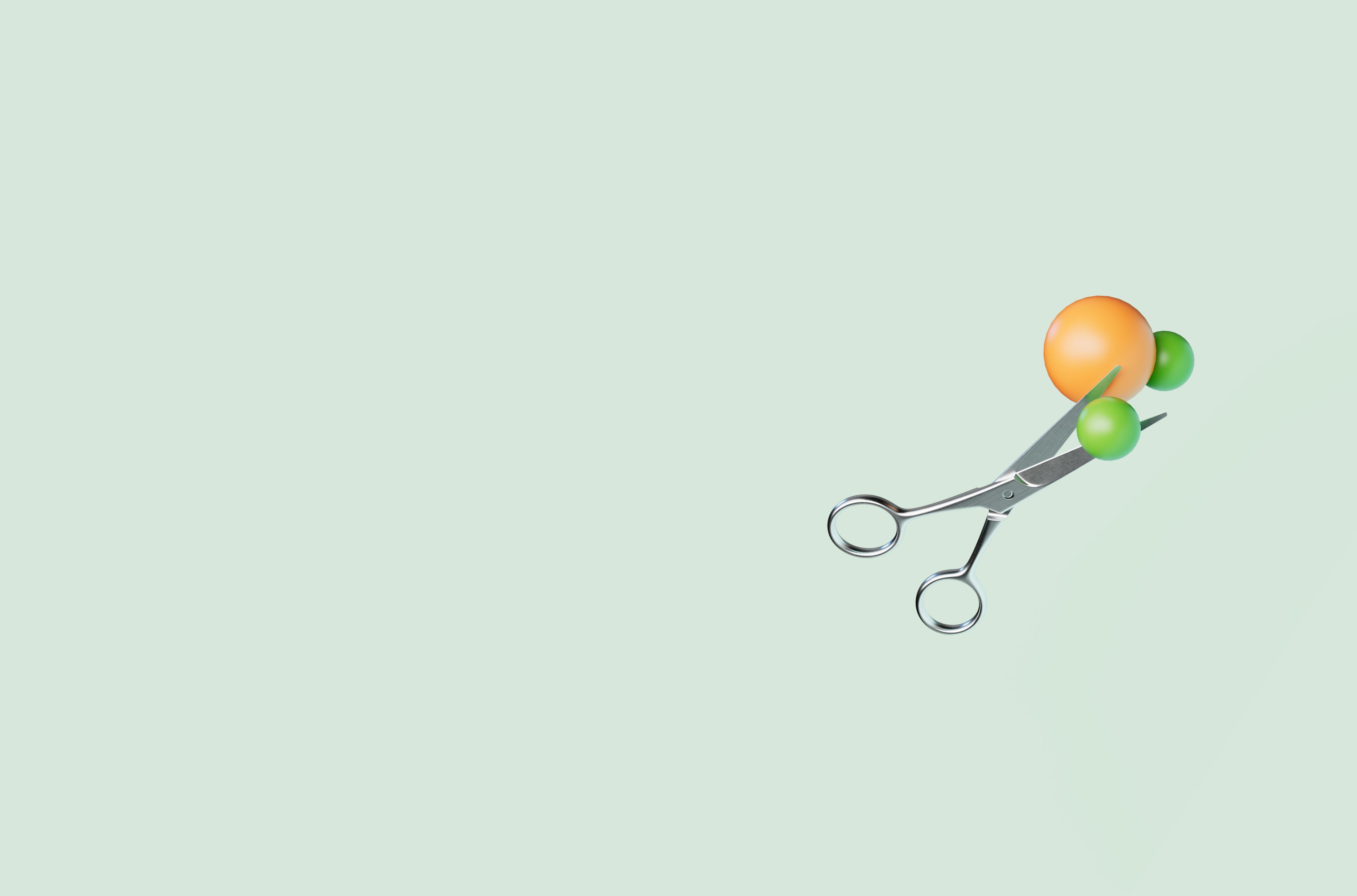




SPLITTING WATER
Water molecules are split into hydrogen and oxygen atoms using an electrolyzer. Alkaline electrolytic cells and proton exchange membrane cells are the two most common water-splitting technologies.
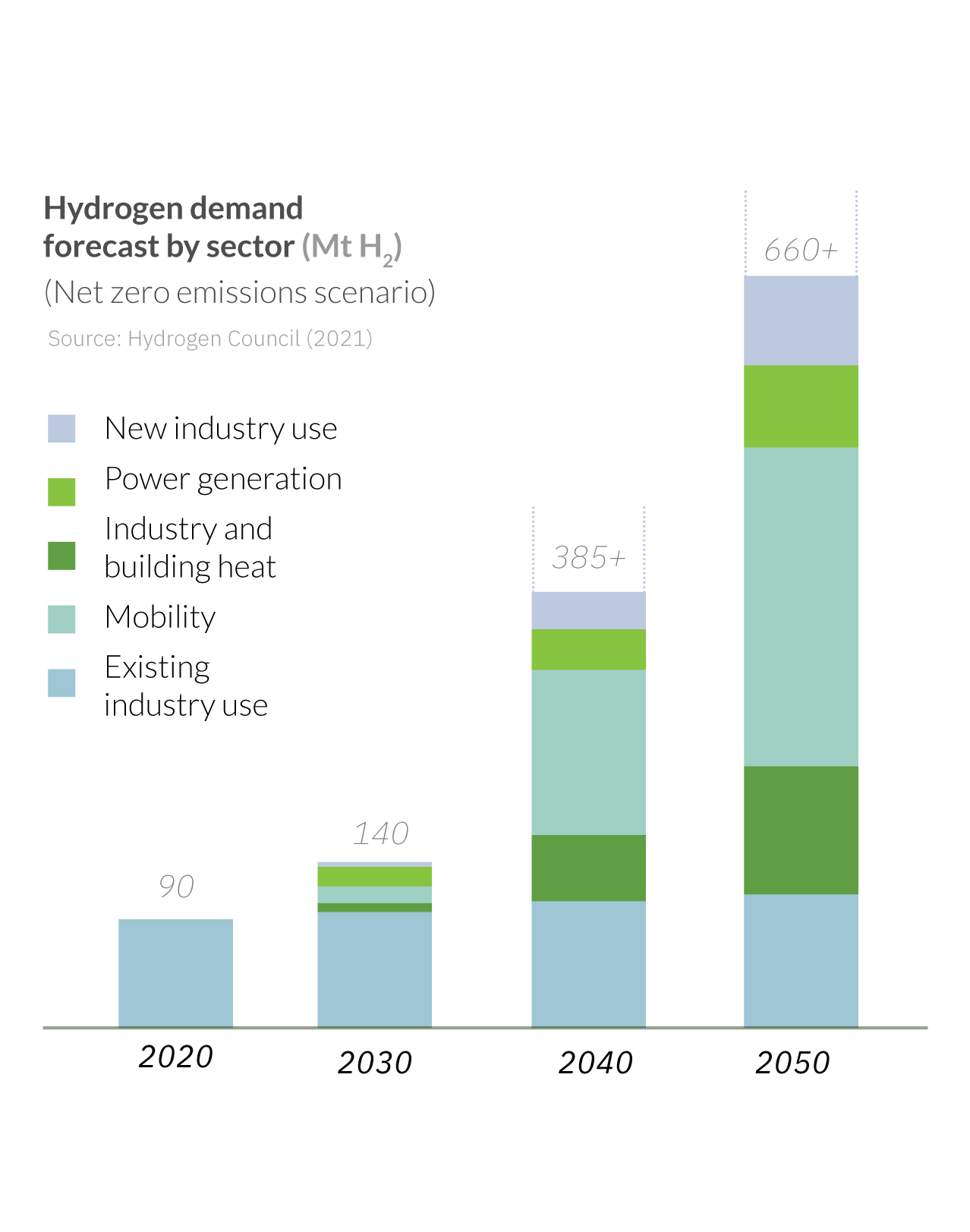
EVER-INCREASING DEMAND
With several countries and industries setting net zero emissions targets by 2050, green hydrogen will play a vital role in their decarbonization efforts, complementing other measures such as direct electrification and other energy efficiency initiatives.
Converting hydrogen fuel into a viable source of energy for everyday power usage seems the simplest piece of the hydrogen power puzzle. The ability to produce power from hydrogen has been studied for centuries, but the prohibitive cost of producing pure hydrogen has prevented its large-scale use until now. The ever-decreasing costs of renewable energy and electrolysis technology mean that hydrogen is becoming competitive with conventional power generation, which is why hydrogen represents one of the most enticing potential energy technologies that will help us finally transition from and ultimately relinquish fossil fuels.
Michael Cusak, Xavier Pita
Research Communication, KAUST